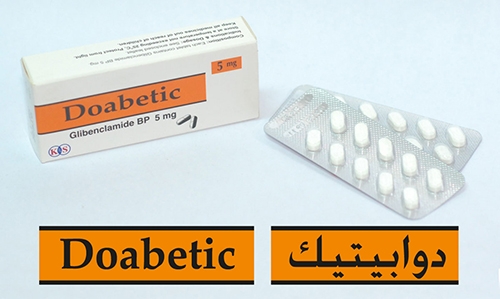
Bahrain withdraws Doabetic from shelves
Health authorities yesterday warned of a Kuwaiti-produced diabetic medicine and called for its immediate withdrawal from local pharmacies.
“National Health Regulatory Authority (NHRA) wishes to advise the public to stop using Doabetic 5 mg (Glibenclamide) Tablet manufactured by Kuwai- Saudi Pharmaceutical Industries Co/ Kuwait,” NHRA said in an announcement yesterday.
The statement read, “Based on the Gulf Central Committee for Drug Registration (GCC-DR) resolution, meeting number 75 held in Dubai/ United Arab of Emirates, the committee decided to suspend the registration of Doabetic as it is failed to comply with the innovator bioequivalence criteria.”
NHRA confirmed that it had taken several actions to ensure the safety of patients. The authoritysuspended the registration license of the medicine, withdrew all the stock from the market and stopped selling and distribution of the medicine in the market.
Related Posts