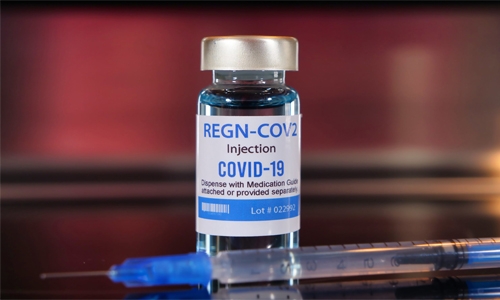
Bahrain approves new drug to treat ‘mild’ COVID cases
TDT | Manama
The Daily Tribune – www.newsofbahrain.com
The National Health Regulatory Authority (NHRA) has approved “REGN-COV2” for emergency use, a new drug by Regeneron in collaboration with F. Hoffmann-La Roche, for the treatment of mild to moderate COVID-19 cases.
REGN-COV2 contains a combination of Casirivimab and Imdevimab, which are drugs called “monoclonal antibodies”, that are designed to block viral attachment and entry into human cells to neutralise the virus.
This drug has been added to the list of approved drugs by the Kingdom of Bahrain for the treatment of COVID-19 using antibody testing technology.
Related Posts